

User-Centered Design:
We prioritize usability and safety from the start, using contextual research, formative studies, and human factors engineering to ensure that the final product meets real-world clinical and patient needs.
Cross-Functional Expertise:
Our multidisciplinary team includes experts in industrial design, mechanical and electrical engineering, embedded systems, software development, quality assurance, and regulatory affairs allowing us to handle every phase from concept through commercialization.
Rapid Prototyping and Iteration:
On-site prototyping labs and usability studios enable us to quickly test ideas and refine designs based on real user feedback, reducing development time and risk.
Compliance and Risk Mitigation:
Through continuous risk analysis and traceability, we ensure products are not only innovative but also safe, effective, and compliant.
Phase-Gated Process:
Our development model follows a structured, phase-gated approach that includes:
Phase 1: Research (user needs, regulatory strategy, feasibility)
Phase 2: Development (design inputs, architecture, early prototypes)
Phase 3: Refinement & Verification (design outputs, verification, risk updates)
Phase 4: Finalization & Validation (validation, transfer to manufacturing, submission support)
Manufacturing Liaison and Transfer:
We support design for manufacturing (DFM) and provide full documentation packages for seamless tech transfer to manufacturing partners, reducing time to market.
Regulatory-Ready Documentation:
We operate under an FDA compliant Quality Management System. Our process aligns with FDA 21 CFR Part 820, ISO 14971, IEC 62366, and other global standards, ensuring traceability, risk management, and documentation for regulatory submission.
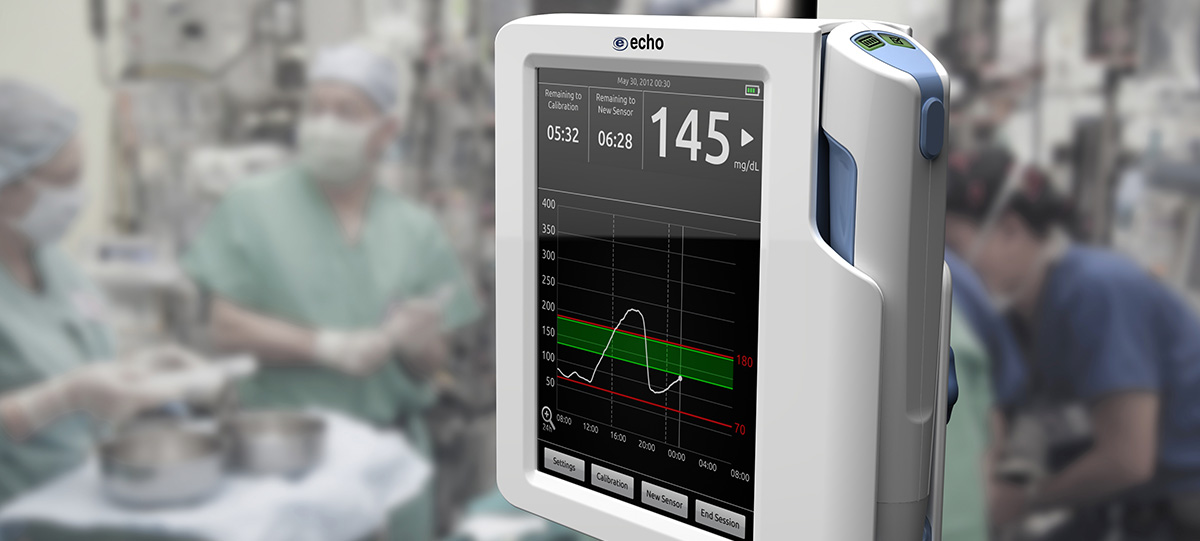

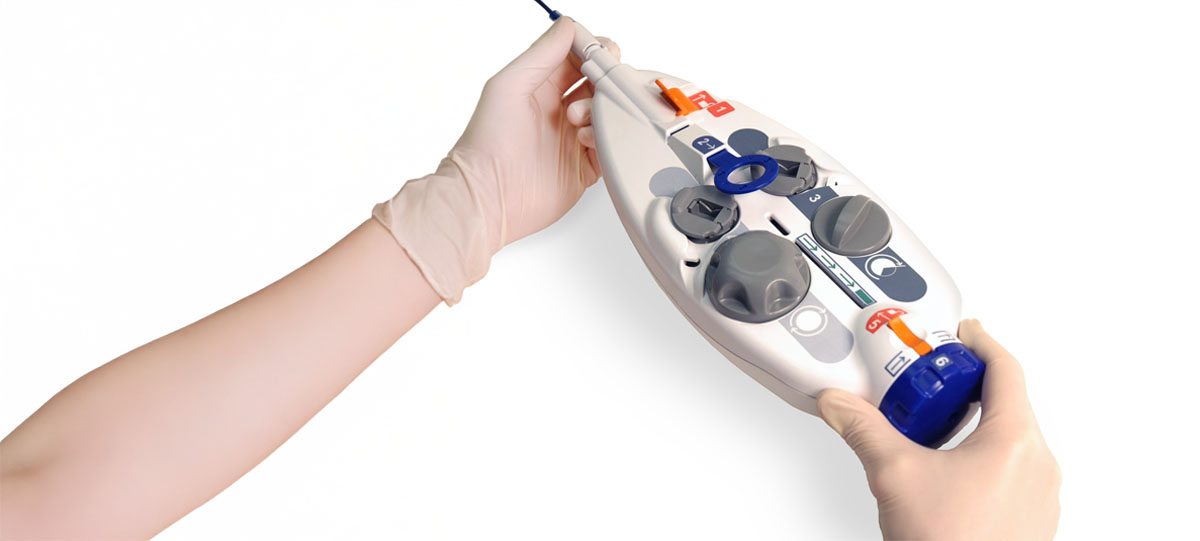

We have a case study available showing the process we used to update this medical cart…
Eclipse Product Development Corp.